CBSE Class 10 Science Important Chapter “Carbon and its Compound” important topic such as definition, allotropes of carbon, the occurrence of carbon, Carbon covalent bond discussion in this chapter.
Hi Everyone, Welcome To CBSE Digital Education. Today We Are Going To Discuss A Interesting Topic About Carbon and its Compound for Class 10. CBSE Digital Education provides all important information regarding Carbon and its Compound Notes for Class 10. Read this complete article till the end.
Complete Explanation of Carbon and its compound for Class 10
Carbon is non-metal. The name of carbon is derived from the Latin word ‘Carbo’ which means coal. The amount of carbon present in the atmosphere and the earth’s crust and is very very small.
- It has the highest catenation power.
- It formed a covalent bond.
- The atomic number of carbon is 6.
- Atomic mass of carbon, A = P + N =12
- The valence electron of carbon is 4.
- The valency of carbon is 4.
- Carbon belongs to fourteen groups in the periodic table.
- Structure of carbon = Tetravalent.
- A carbon compound called organic compounds.
Carbon always forms covalent bonds
Carbon atoms can achieve the noble gas electron arrangement only by the sharing of electrons, therefore, carbon always forms covalent bonds.
Carbon is Tetravalent: Carbon is normally tetravalent, meaning it makes four bonds to other atoms.
An example is a methane (CH4): the tetravalent carbon atom forms a covalent bond with four hydrogen atoms. The carbon atom is also known as tetravalent because it forms 4 covalent bonds.
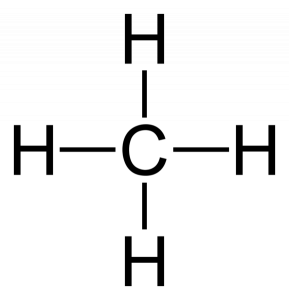
Self combination property of carbon
The property of self combination of carbon atoms to form long chains is known as catenation.
Occurrence of carbon
Carbon occurs in nature in the ‘free state’ (as an element) as well as in the ‘combined state’ (in the form of compounds with other elements).
- In Free State: carbon occurs in the natures in the forms of diamond, graphite, and buckminsterfullerene. Another naturally occurring form of carbon called Graphene has been discovered recently. The only small amount of carbon occurs as a free element in the earth’s crust.
- In the combined state, carbon occurs in nature in the form of compounds such as Carbon dioxide, Carbonates (like limestone, marble, and chalk), Fossil fuels like coal, petroleum, and natural gas.
Allotropes of carbon
The various physical forms in which an element can exist are called allotropes of the elements. The four allotropes of carbon are:
- Diamond
- Graphite
- Buckminsterfullerene
- Graphene
Diamond
Diamond is the hardest substance having extraordinary brilliance. If we burn diamonds in oxygen, then only carbon dioxide gas is formed and nothing is left behind. This shows that the diamond is made up of carbon only.

- It is the hardest and strongest allotrope of carbon.
- It is a bad conductor of electricity due to the absence of free electrons.
- It is used in glass cutting and paper cutting. A black diamond is used in mirror cutting.
- The melting point of the diamond is 4726.
- The density of the diamond is 5.3-5.4 g/c m 3.
- The hybridization of the diamond is s p 3.
- The rigid structure of the diamond makes it a very hard substance.
- Diamonds are used for making jewelry.
- Sharp-edged diamonds are used by eye surgeons as a tool to remove the cataract from the eyes with great precision.
- Diamond can be made artificially by subjecting pure carbon to very high temperatures and pressure.
Graphite
- It is a grayish-black opaque substance. Graphite is lighter than a diamond. When we burn graphite in oxygen, then only carbon dioxide gas is formed and nothing is left behind. This shows that graphite is made up of carbon only.

- It is also called a black lead.
- It is a good conductor of electricity.
- The melting point of graphite is 3527
- The density of graphite is 2.23 g/c m 3.
- The hybridization of graphite is s p 2.
- It is used in pencil lead.
- Graphite is used as a lubricant.
- It is used in a nuclear reactor as a control rod.
- It is used for making carbon electrodes or graphite electrodes in dry cells and electric arcs.
Buckminsterfullerene
Buckminsterfullerene is an allotrope of carbon-containing clusters of 60 carbon (C60) atoms joined together to form spherical molecules.
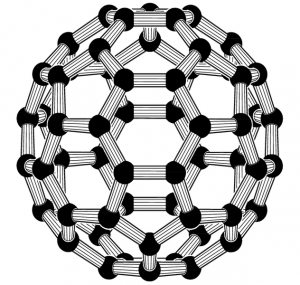
- It is also called buckyball/fullerene.
- It is a good conductor of electricity.
- The melting point of buckminsterfullerene is 600
- The hybridization of buckminsterfullerene is s p 2.
- It is used in nanotechnology / nano-tubes.
- It is used for drug delivery in human beings.
- Formula of buckminsterfullerene is C 60.
- It is dark solid at room temperature.
Graphene
It is the latest discovered allotrope of carbon.
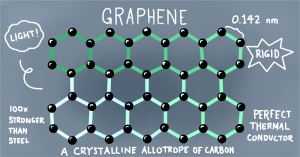
- Graphene is discovered by Gill.
- It is a good conductor of electricity.
- The hybridization of graphene is s p 2.
- The melting point of graphene is 3000
- It is used in making mobile touch screens, LED TV touch, and bulletproof jackets.
I hope you like this article about Carbon and its Compound Notes. If you want to ask any queries regarding Carbon and its Compound then message us in the comment section, and we will reply to you soon.
Good morning sir,
This method of teaching is very nice. First ,all students will look at the pictures or models of that topics and also Teacher will show the proper teaching materials to the pupils..That is easy for better understanding .